23+ Calculate Zeff For A Valence Electron In An Oxygen Atom.
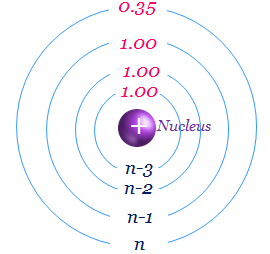
Calculate the for the valence electron in an oxygen. Use Slaters Rules O 1s2 2s 2p6 So shielding S 035 x 5 085 x 2 345 Zeff Z.
8 4 Electron Configurations Valence Electrons And The Periodic Table Chemistry Libretexts
Calculate the effective nuclear charge on a valence electron in a bromine atom.
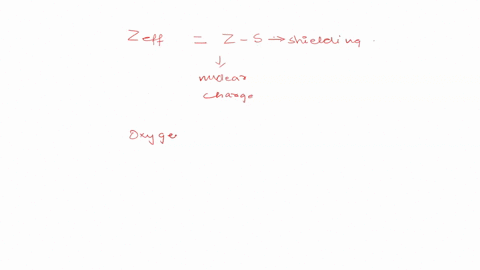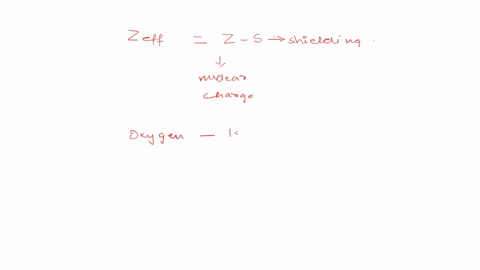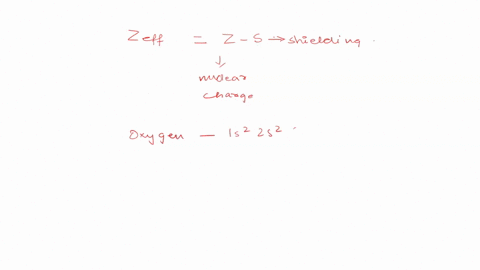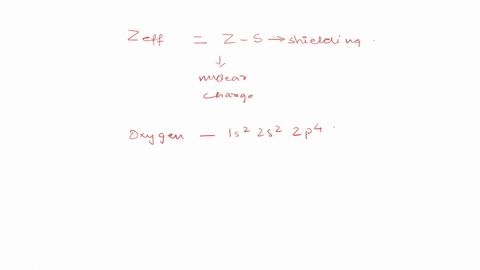
. You have been asked to prepare. An atom is always more stable when it has 8 valence electrons. Jpdvolleybal1326 jpdvolleybal1326 11172017 Chemistry College.
We store cookies data for a seamless user experience. The effective nuclear charge experienced by a valence electron in an O atom is 455. Express your answer numerically.
Calculate Zeff for the following. Calculate zeff for a valence electron in an oxygen atom. Besides the formula for calculating the effective nuclear charge of a single electron is as follows.
7 35 - 28 Bromine has an atomic number of 35. There are 35 protons and 35 electrons in a bromine. 2 Jordan Artzy 4 y Effective Nuclear Charge is equal to the atomic number protons subtracted by the shielding electrons or valence.
An atom is always more stable when it has 8 valence electrons. Calculate Zeff for a. Part A Calculate Zeff for a valence electron in an oxygen atom.
Express your answer numerically. Zeff Z S 8 345 455. Zeff 345 Submit Previous Answers X.
View Available Hint s IVO ΟΙ ΑΣΦ. Calculato Zeff for a valence electron in an oxygen atom. 2 Answers Use Slaters Rules O 1s2 2s 2p6 Therfore shielding S 035 x 5 085 x 2 345 Zeff Z S 8 345 455 The effective nuclear.
The effective nuclear charge for oxygen atom is 455. Up to 256 cash back Get the detailed answer. Thanks in advance.
Who are you and how did you know that. Calculate the for the valence electron in an oxygen atom. Calculate Zeff for a valence electron in an oxygen atom.
View Available Hints vo ΑΣφ Zer_eff 6 Submit Previous Answers X Incorrect. To determine the value for S start by writing out the electron configuration for the atom. Calculate Zeff for a valence electron in an oxygen atom.
The magnesium has 2 valence electron while the oxygen misses 2 electron to complete its octet. Calculate Zett for the 4s electron in a copper atom Cu. The electron configuration for Cu is 1s22s22p63s23p63d104s1.
Express your answer numerically. Up to 256 cash back The number of electrons in the Noble Gas just before the atom of interest will be considered equal to the sheilding constant. A A valence electron in an oxygen atom b A valence electron in an iron atom c A 2p electron in an iron atom 7.
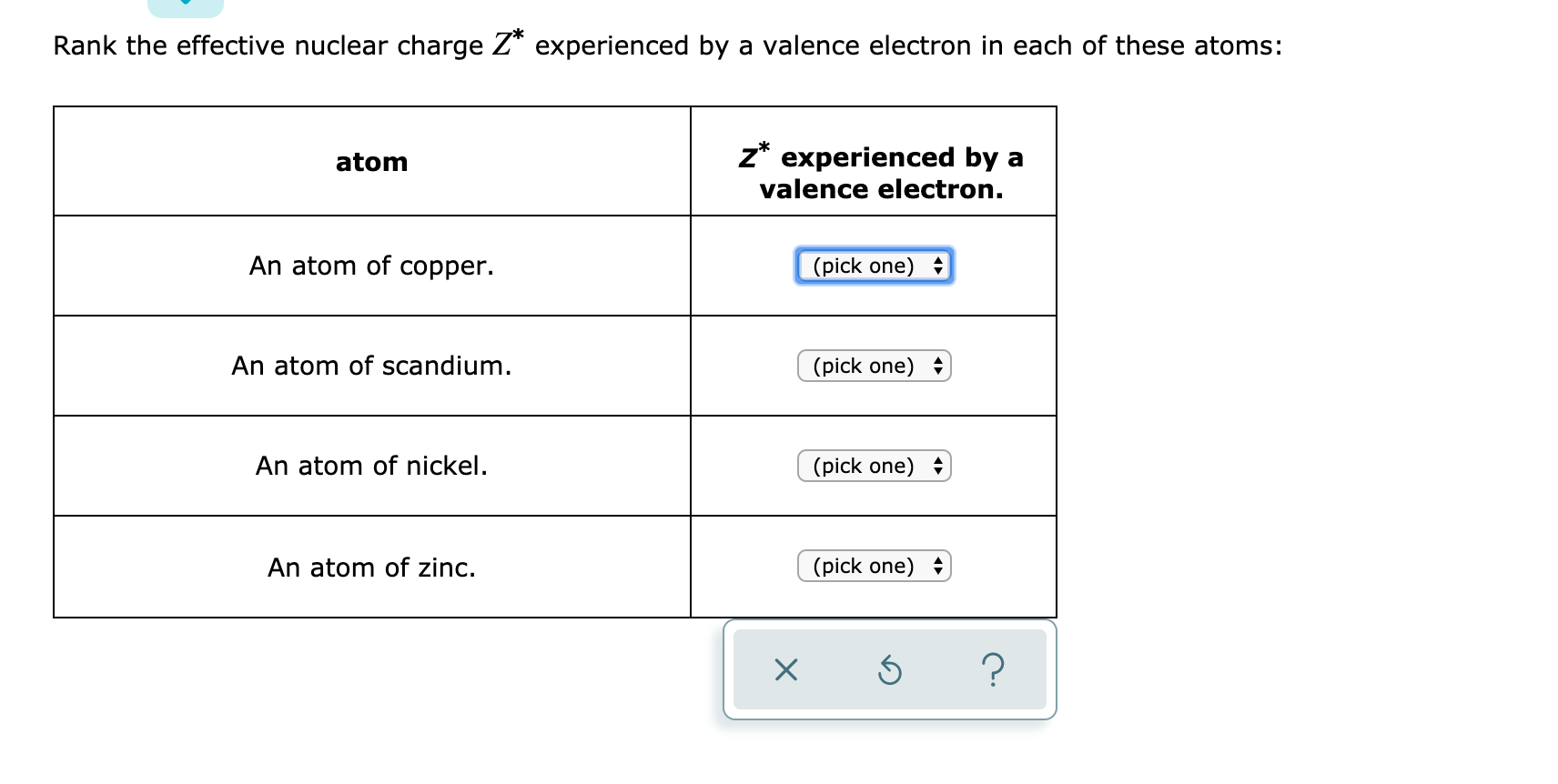
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com

Valence Electrons And The Periodic Table Youtube

Chapter 4 Periodic Trends Of The Elements Ppt Download

How To Find The Effective Nuclear Charge Of Oxygen Quora

Calculate The Effective Nuclear Charge Of The Last Electron In An Atom The Electronic Configuration Is 1s 2 2s 2 2p 6 3s 2 3p 5
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com
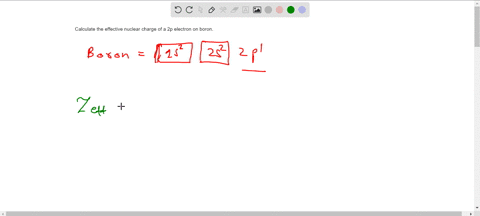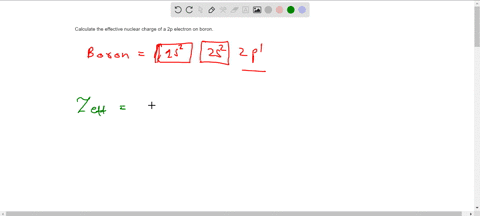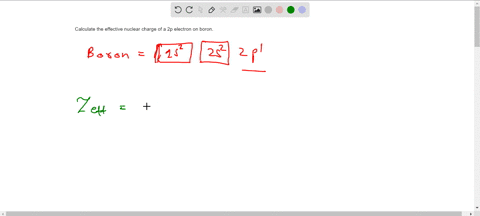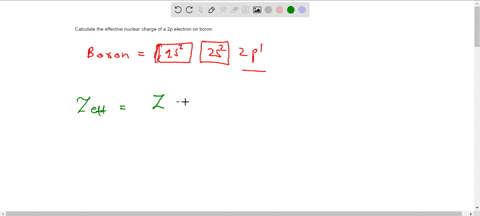
Solved Calculate The Effective Nuclear Charge On A Valence Electron In An Oxygen Atom

Chapter 3 Atomic Structure And Properties
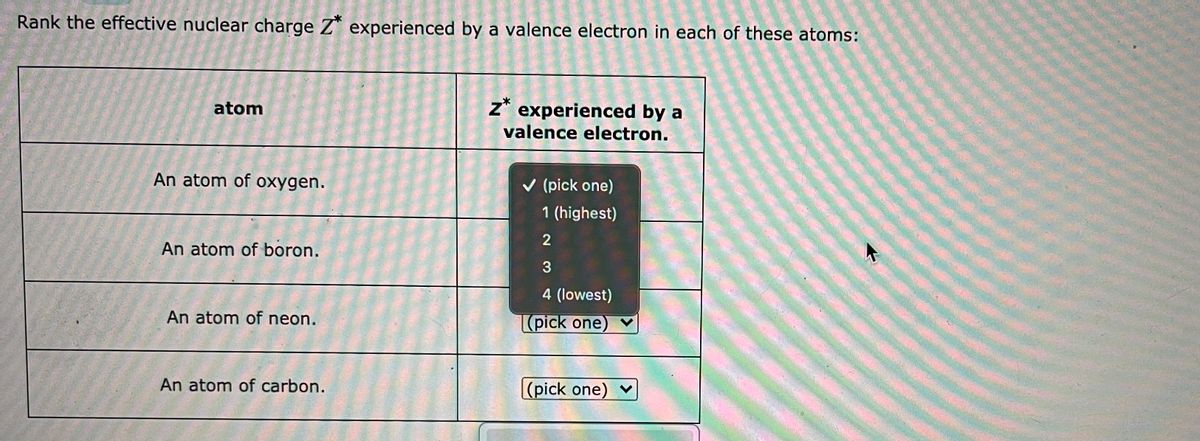
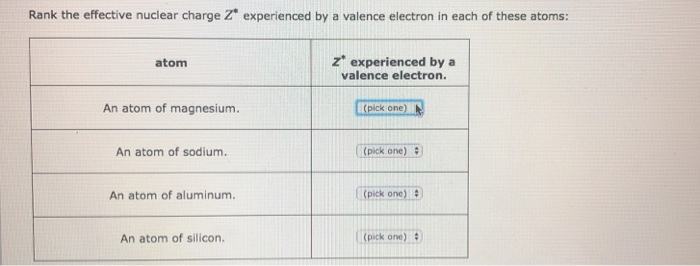
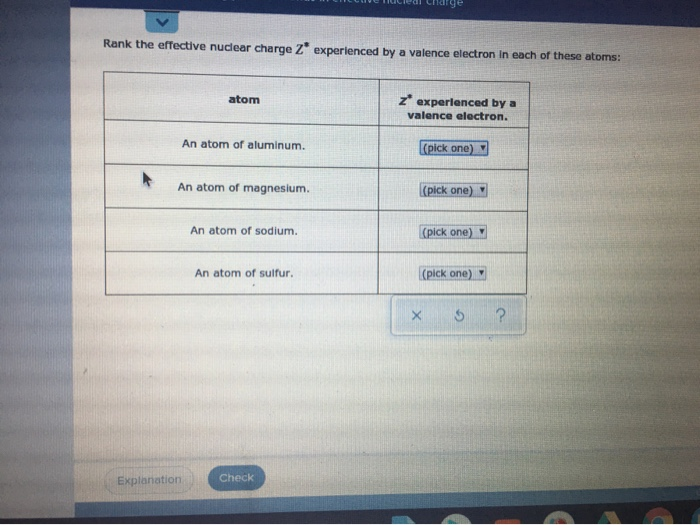
Answered Rank The Effective Nuclear Charge Z Bartleby

Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com

Calculate Zeff For A Valence Electron In An Oxygen Atom Brainly Com

Solved 3 Calculate Zeff For Oxygen Atom On Outermost Shell Chegg Com

Solved Calculate The Effective Nuclear Charge On A Valence Electron In An Oxygen Atom
Shielding Electrons Slater S Rule Effective Nuclear Charge
Answered Rank The Effective Nuclear Charge Z Bartleby

This Question Consists Of Legal Proposition S Principle S Hereinafter Referred To As Principle And Facts Such Principles May Or May Not Be True In The Real And Legal Sense Yet You Have To Conclusively
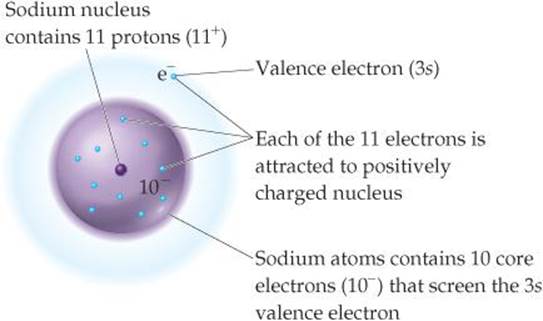
Effective Nuclear Charge Periodic Properties Of The Elements Chemistry The Central Science